What Happens To The Shape Of Matter When Changed From Gas To Solid
You would take observed changing states of matter when ice cubes melt from solid into liquid water or when h2o boils into vapor, but have you lot wondered why substances change class? Changing states of matter occur when thing loses or absorbs energy. When a substance absorbs free energy the atoms and molecules move more than speedily and this increased kinetic energy pushes particles far enough, that they change grade. This free energy is unremarkably heat or thermal energy. In this article, let us sympathize the science behind the changing states of thing.
You might want to read the following articles for a deeper agreement of the topic.
- Iii States of Matter
- Concrete and Chemical Changes
What are Changes of Country?
A modify of country is a concrete change in a matter. They are reversible changes and practice not involve any changes in the chemical makeup of the affair. Common changes of the state include melting, freezing, sublimation, degradation, condensation, and vaporization. These changes are shown in the figure given below.
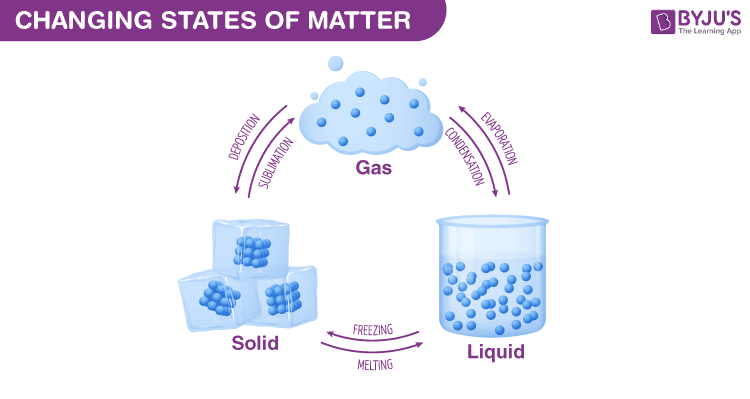
Why do Phase Changes Occur?
When temperature or pressure change of a system occurs, phase changes occur. When the temperature or pressure increases, the interaction between the molecules increases. Similarly, when the temperature decreases, information technology is easier for molecules and atoms to settle into a more rigid structure.
FreezingMeltingVaporizationCondensationSublimationQuestions
Changes Between Liquids and Solids
How would you make ice cubes in a tray? First, you would fill the tray with water from a tap. Then you would place the tray in the freezer compartment of a fridge. The freezer is very cold. What happens side by side?
Freezing
Oestrus transfer occurs between the warmer tray and the colder air in the freezer. The warm water loses heat to the cold air in the freezer. This oestrus transfer occurs until no energy is available for the particles to slide past each other. This forces them to remain in fixed positions, locked in identify by the forcefulness of attraction betwixt them. This way liquid water is changed into solid ice. The procedure of liquid h2o changing to solid ice is termed every bit freezing. The temperature at which it occurs is known as the freezing betoken.
Melting
If you took out the water ice cubes from the freezer and placed them in a warm room, the ice would absorb free energy from the warmer air around them. This absorbed free energy would facilitate them to overcome the strength of allure holding them together, enabling them to slip out of the fixed position that they held every bit ice. The procedure in which a solids modify to a liquid is called melting. The melting betoken is the temperature at which a solids change to a liquid.
Sentry the video below to conspicuously understand why water changes to solid when the temperature is reduced and to a gas when the temperature is increased?

Changes Between Liquids and Gases
If you fill a pot with cold tap water and heat information technology on a hot stovetop, the water heats up. Estrus energy travels from the stovetop to the pot, and the water absorbs the energy from the pot. What happens to the h2o next?
Vaporization
If the water is hot plenty, information technology starts to boil. Bubbles of water vapor are formed in the boiling water. This happens equally particles of liquid water gain enough energy to completely overcome the force of attraction betwixt them and modify to the gaseous country. The bubbles rise through the water and escape from the pot as steam. The process in which a liquid boils and changes to a gas is called vaporization. The temperature at which a liquid boils is its humid point.
Condensation
When y'all take a hot shower in a closed bathroom, the mirror is likely to fog upwardly. You may wonder why does this happen? Some hot h2o from the shower evaporates and when it comes in contact with cooler surfaces such every bit the mirror, it cools and loses energy. The cooler h2o particles no longer have the energy to overcome the forces of attraction between them. They come up together and form aerosol of liquid water. This process in which a gas changes to liquid is known as condensation.
Changes Between Solids and Gases
Solids that change to gas passes through the liquid state get-go. Notwithstanding, sometimes solids modify directly to gases and skip the liquid land. The contrary tin can besides occur. Sometimes gases change straight to solids.
Sublimation
The process in which solids directly change to gases is known as sublimation. This occurs when solids absorb plenty energy to completely overcome the forces of attraction betwixt them. Dry out ice is an instance of solids that undergo sublimation.
| Five Changes of State | ||||
| Melting | Freezing | Evaporation | Condensation | Sublimation |
| The process past which a substance changes from the solid stage to the liquid phase is known every bit melting. | The process past which a substance changes from the liquid phase to the solid stage is known as freezing. | The process by which a substance changes from the liquid phase to the gaseous phase is known as evaporation. | The procedure by which a substance changes from the gaseous phase to the liquid stage is known as condensation. | The transition of the solid phase to the gaseous stage without passing the intermediate liquid phase is known as sublimation. |
Conclusion
It will interest you to know that every object in being undergoes a state change. It is only a question of the amount of heat supplied to the substance. If you supply plenty estrus, everything on this planet can be fabricated to change its state. The thing is though not every substance has to follow the solid-liquid-gas path. Some substances tin naturally change from their solid-state to their gaseous land without entering the liquid state. This phenomenon is known as Sublimation. Examples of sublimation are, the element Iodine, Dry ice (solid CO2) and high-quality coal which at loftier-temperature burns and sublimates into vapour.
Stay tuned to BYJU'Southward to acquire more interesting concepts like changing states of affair with the assistance of engaging video lessons.
Frequently Asked Questions – FAQ's
When solids reach their melting point, what exercise they become?
Solids transform into liquid when they reach their melting point.
What is the boiling indicate?
Boiling point is defined as a temperature at which a pure liquid changes into a gas.
What is the melting point?
The melting point is divers as the temperature at which the solid starts to cook.
What is the process in which solids directly transform into a gas?
Ans: Sublimation is defined every bit the process in which the solid-land changes to a gaseous state without changing into a liquid land.
What is evaporation?
Ans: When the liquid gets converted to gas at all the temperatures, it is known equally evaporation.
The globe around yous is filled with interesting facts similar these. Learn all nearly them at BYJU'S.
Source: https://byjus.com/physics/changing-states-of-matter/
Posted by: tobinmors1941.blogspot.com


0 Response to "What Happens To The Shape Of Matter When Changed From Gas To Solid"
Post a Comment